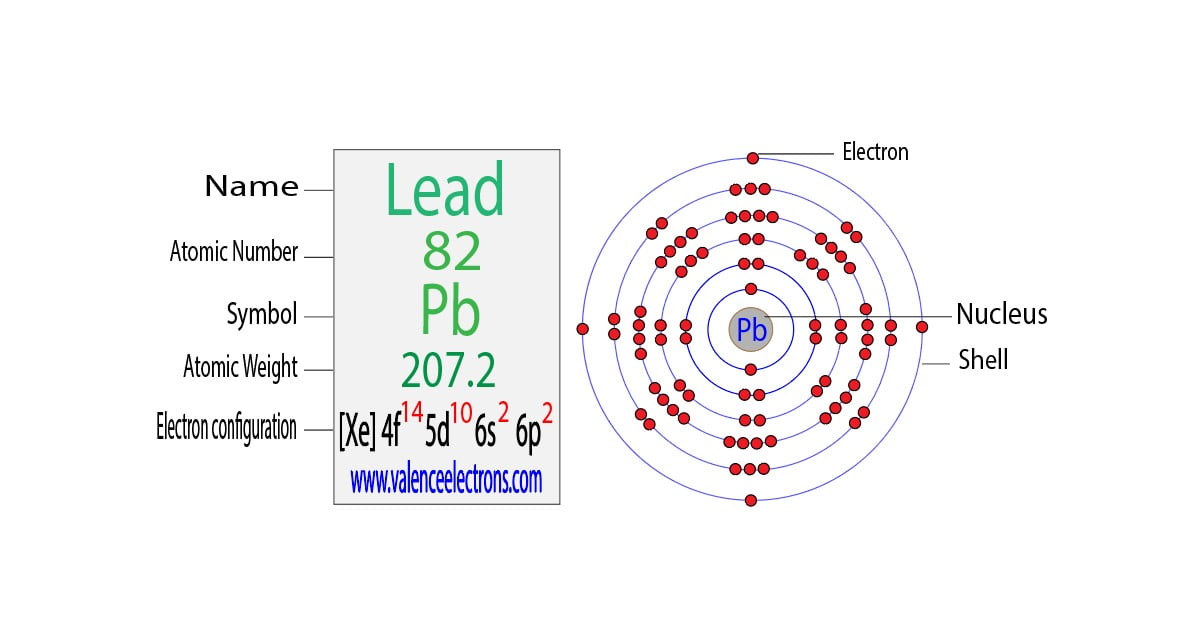
Neutral Bromine Atom Have Valence Electrons . 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. valence electrons are the electrons present in the outermost shell of an atom. What is the electron configuration of bromine? valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. Bromine has an electron configuration of. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. valence electrons found in the s and p orbitals of the highest energy. You can easily determine the number of valence electrons an atom can.
from valenceelectrons.com
Bromine has an electron configuration of. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. valence electrons found in the s and p orbitals of the highest energy. What is the electron configuration of bromine? valence electrons are the electrons present in the outermost shell of an atom. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. You can easily determine the number of valence electrons an atom can.
Complete Electron Configuration for Bromine (Br, Br ion)
Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. You can easily determine the number of valence electrons an atom can. valence electrons found in the s and p orbitals of the highest energy. What is the electron configuration of bromine? valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. valence electrons are the electrons present in the outermost shell of an atom. Bromine has an electron configuration of. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Neutral Bromine Atom Have Valence Electrons valence electrons found in the s and p orbitals of the highest energy. valence electrons are the electrons present in the outermost shell of an atom. What is the electron configuration of bromine? when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may. Neutral Bromine Atom Have Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Neutral Bromine Atom Have Valence Electrons valence electrons found in the s and p orbitals of the highest energy. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. valence electrons are the electrons present in the outermost shell of an atom. Bromine has an electron configuration of. valence electrons are the electrons. Neutral Bromine Atom Have Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. valence electrons found in the s and p orbitals of the highest energy.. Neutral Bromine Atom Have Valence Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Neutral Bromine Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. What is the electron configuration of bromine? You can easily determine the number of valence electrons an atom can. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full. Neutral Bromine Atom Have Valence Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Bromine has an electron configuration of. What is the electron configuration of bromine? valence electrons. Neutral Bromine Atom Have Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons present in the outermost shell of an atom. valence electrons found in the s and p orbitals of the highest energy. valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. 93 rows you may assume the valences. Neutral Bromine Atom Have Valence Electrons.
From diagramdataconfusion.z22.web.core.windows.net
Electron Dot Diagram For Bromine Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons present in the outermost shell of an atom. What is the electron configuration of bromine? You can easily determine the number of valence electrons an atom can. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may assume the. Neutral Bromine Atom Have Valence Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Neutral Bromine Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. valence electrons are the electrons present in the outermost shell of an atom. Bromine has an electron configuration of. What is the electron configuration of bromine? valence electrons are the electrons that reside in the outermost. Neutral Bromine Atom Have Valence Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Neutral Bromine Atom Have Valence Electrons What is the electron configuration of bromine? You can easily determine the number of valence electrons an atom can. Bromine has an electron configuration of. valence electrons are the electrons present in the outermost shell of an atom. valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most. Neutral Bromine Atom Have Valence Electrons.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. valence electrons found in the s and p orbitals of the highest energy. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.. Neutral Bromine Atom Have Valence Electrons.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons present in the outermost shell of an atom. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. You can easily determine the number of valence electrons an atom can. valence electrons are the electrons that reside in the outermost energy level of. Neutral Bromine Atom Have Valence Electrons.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry Neutral Bromine Atom Have Valence Electrons You can easily determine the number of valence electrons an atom can. valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. valence electrons are the electrons present in the outermost shell of an atom. What is the electron configuration of bromine? valence electrons. Neutral Bromine Atom Have Valence Electrons.
From brainly.in
bromine valence electrons, Brainly.in Neutral Bromine Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. valence electrons are the electrons present in the outermost shell of an atom. valence electrons are. Neutral Bromine Atom Have Valence Electrons.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. valence electrons found in the s and p orbitals of the highest energy. What is. Neutral Bromine Atom Have Valence Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Neutral Bromine Atom Have Valence Electrons valence electrons found in the s and p orbitals of the highest energy. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine has an electron configuration of. valence electrons are the electrons present in the outermost shell of an atom. when forming ions,. Neutral Bromine Atom Have Valence Electrons.
From dxosghbuq.blob.core.windows.net
Electron Dot Structure For Bromine at Teresa Davis blog Neutral Bromine Atom Have Valence Electrons valence electrons found in the s and p orbitals of the highest energy. You can easily determine the number of valence electrons an atom can. Bromine has an electron configuration of. valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. when forming ions,. Neutral Bromine Atom Have Valence Electrons.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Electron Dot Symbol For Bromine Neutral Bromine Atom Have Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. valence electrons found in the s and p orbitals of the highest energy. valence electrons are. Neutral Bromine Atom Have Valence Electrons.
From www.slideserve.com
PPT The Parts of an Atom PowerPoint Presentation, free download ID Neutral Bromine Atom Have Valence Electrons valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Neutral Bromine Atom Have Valence Electrons.